Top 10 Publikationen
Wang B, Briegel J, Krueger WA, Draenert R, Jung J, Weber A, Bogner J, Schubert S, Liebchen U, Frank S, Zoller M, Irlbeck M, Ney L, Weig T, Hinske L, Niedermayer S, Kilger E, Mohnle P, Grabein B. 2022. Ecological effects of selective oral decontamination on multidrug-resistance bacteria acquired in the intensive care unit: a case-control study over 5 years. Intensive Care Med 48:1165-1175.
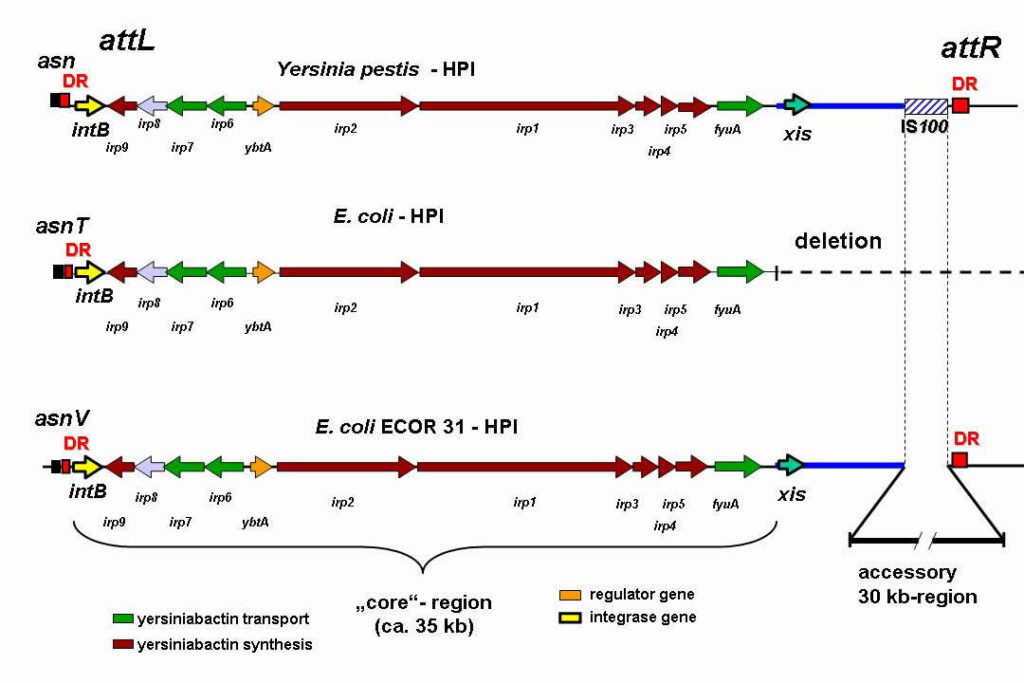
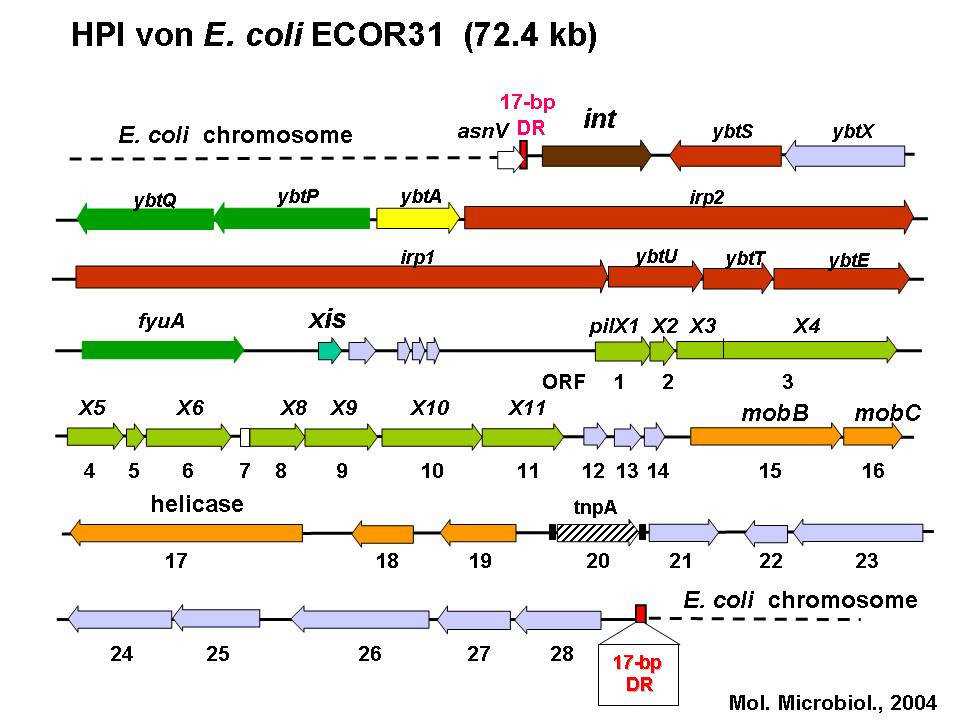
Rehm N, Wallenstein A, Keizers M, Homburg S, Magistro G, Chagneau CV, Klimek H, Revelles O, Helloin E, Putze J, Nougayrede JP, Schubert S, Oswald E, Dobrindt U. 2022. Two Polyketides Intertwined in Complex Regulation: Posttranscriptional CsrA-Mediated Control of Colibactin and Yersiniabactin Synthesis in Escherichia coli. mBio 13:e0381421.
Pilatz A, Veeratterapillay R, Dimitropoulos K, Omar MI, Pradere B, Yuan Y, Cai T, Mezei T, Devlies W, Bruyere F, Bartoletti R, Koves B, Geerlings S, Schubert S, Grummet J, Mottet N, Wagenlehner F, Bonkat G. 2021. European Association of Urology Position Paper on the Prevention of Infectious Complications Following Prostate Biopsy. Eur Urol 79:11-15.
Schubert SP, R.; Gatermann, S.; Fünfstück, R.; Naber, K.G.; Schimanski, S.; Wagenlehner, F. 2020. Mikrobiologisch-infektiologische Qualitätsstandards (MiQ) - Teil 2, Harnwegsinfektionen, 3. ed, vol 02. Elsevier, Munich, Germany.
Pilatz A, Veeratterapillay R, Dimitropoulos K, Omar MI, Pradere B, Yuan Y, Cai T, Mezei T, Devlies W, Bruyere F, Bartoletti R, Koves B, Geerlings S, Schubert S, Grummet J, Mottet N, Wagenlehner F, Bonkat G. 2020. European Association of Urology Position Paper on the Prevention of Infectious Complications Following Prostate Biopsy. Eur Urol doi:10.1016/j.eururo.2020.10.019.
Schubert S, Kostrzewa M. 2017. MALDI-TOF MS in the Microbiology Laboratory: Current Trends. Curr Issues Mol Biol 23:17-20.
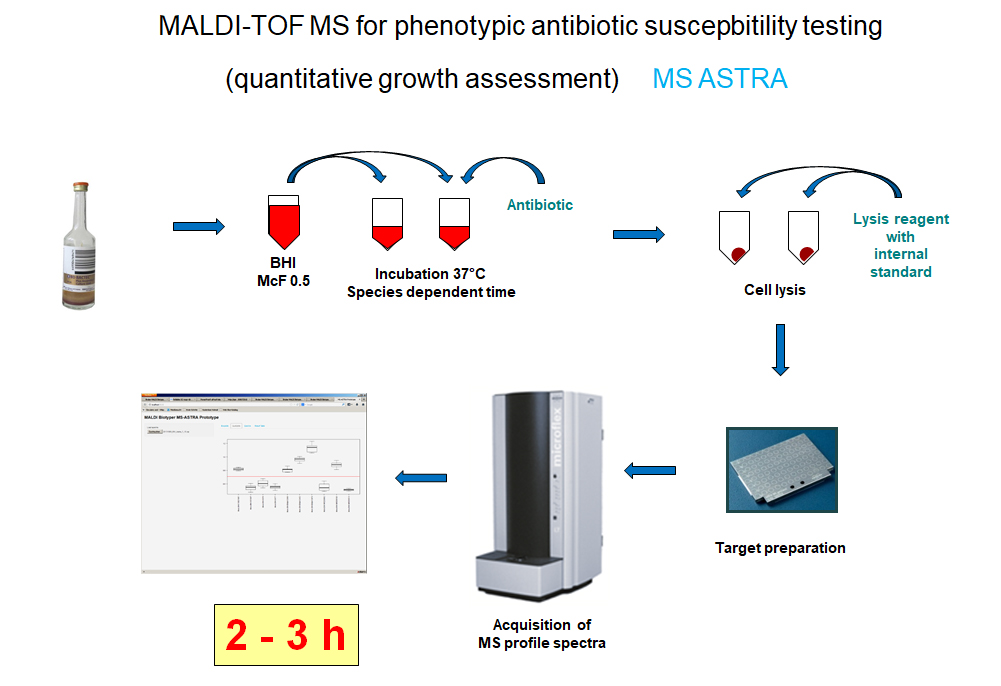
Jung JS, Hamacher C, Gross B, Sparbier K, Lange C, Kostrzewa M, Schubert S. 2016. Evaluation of a Semiquantitative Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Method for Rapid Antimicrobial Susceptibility Testing of Positive Blood Cultures. J Clin Microbiol 54:2820-2824.
Magistro G, Magistro C, Stief CG, Schubert S. 2018. A simple and highly efficient method for gene silencing in Escherichia coli. J Microbiol Methods 154:25-32.
Galardini M, Clermont O, Baron A, Busby B, Dion S, Schubert S, Beltrao P, Denamur E. 2020. Major role of iron uptake systems in the intrinsic extra-intestinal virulence of the genus Escherichia revealed by a genome-wide association study. PLoS Genet 16:e1009065.
Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, Stappert D, Wantia N, Rodriguez N, Wagner H, Svanborg C, Miethke T. 2008. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med 14:399-406.