Top 10 Publikationen
Albanese A, Chen HR, Gapp M, Muenchhoff M, Yang HH, Peterhoff D, Hoffmann K, Xiao Q., Ruhle A, Ambiel I, Schneider S, Mejías Pérez, E, Stern M Wratil PR, Hofmann K, Amann L, Jocham L, Fuchs T, Ulivi AF, Besson-Girard S, Weidlich S, Schneider J, Spinner CD, Sutter K, Dittmer D, Humpe A, Baumeister P, Wieser A, Rothenfusser S, Bogner J, Roider J, Knolle P, Hengel H, Wagner R, Laketa V, Facker OT, Keppler OT. Receptor Transfer between immune cells by autoantibody-enhanced, CD32-driven trogocytosis is hijacked by HIV-1 to infect resting CD4 T cells. Cell Rep Med 2024 Apr 16; 5(4):101483. doi: 10.1016/j.xcrm.2024.101483. Epub 2024 Apr 4. https://pubmed.ncbi.nlm.nih.gov/38579727/
Keppler-Hafkemeyer A*, Greil C, Wratil PR, Shoumariyeh K, Stern M, Hafkemeyer A, Ashok D, Hollaus A, Lupoli G, Priller A, Bischof ML, Ihorst G, Engelhardt M, Marks R, Finke J, Bertrand H, Dächert C, Muenchhoff M, Badell I, Emmerich F, Halder H, Spaeth PM, Knolle PA, Protzer U, von Bergwelt-Baildon M, Duyster J, Hartmann TN, Moosmann A, Keppler OT*. Potent high-avidity neutralizing antibodies and T cell responses after COVID-19 vaccination in individuals with B cell lymphoma and multiple myeloma. Nature Cancer (1):81-95 (2023). https://pubmed.ncbi.nlm.nih.gov/36543907/
Wratil PR, Stern M, Priller A, Willmann A, Almanzar G, Vogel E, Feuerherd M, Cheng CC, Yazici S, Christa C, Jeske S, Lupoli G, Vogt T, Albanese M, Mejías-Pérez E, Bauernfried S, Graf N, Mijocevic H, Vu M, Tinnefeld K, Wettengel J, Hoffmann D, Muenchhoff M, Daechert C, Mairhofer H, Krebs S, Fingerle V, Graf A, Steininger P, Blum H, Hornung V, Liebl B, Überla K, Prelog M, Knolle P, Keppler OT*, Protzer U*. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nature Medicine 28(3):496-503 (2022). https://doi.org/10.1038/s41591-022-01715-4
Albanese M*, Ruhle A, Mittermaier J, Mejías-Pérez E, Madeleine Gapp, Linder A, Schmacke NA, Hofmann K, Hennrich AA, Levy DN, Humpe A, Conzelmann K-K, Hornung V, Fackler OT, Keppler OT*. Rapid, efficient and activation-neutral gene editing of polyclonal primary human resting CD4+ T cells allows unprecedented functional analyses. Nature Methods 19(1):81-89 (2022). https://doi.org/10.1038/s41592-021-01328-8
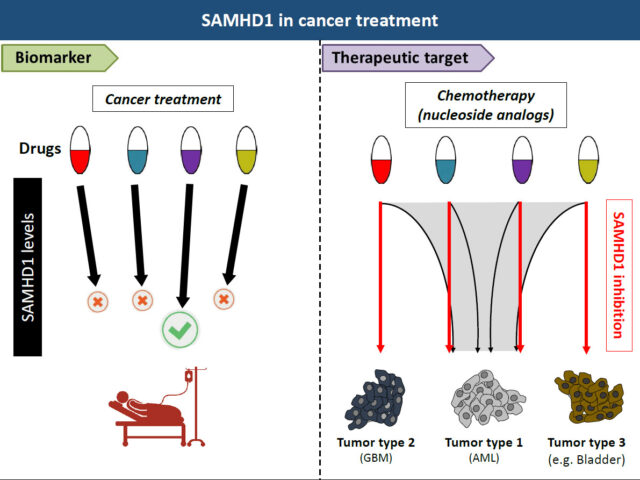
Oellerich T, Schneider C, Thomas D, Knecht KM, Buzovetsky O, Kaderali L, Schliemann C, Bohnenberger H, Angenendt L, Hartmann W, Wardelmann E, Rothenburger T, Mohr S, Scheich S, Comoglio F, Wilke A, Ströbel P, Serve H, Michaelis M, Ferreirós N, Geisslinger G, Xiong Y, Keppler OT*, Cinatl J Jr*. Selective inactivation of hypomethylating agents by SAMHD1 provides a rationale for therapeutic stratification in AML. Nature Communications 2;10(1):3475 (2019). https://doi.org/10.1038/s41467-019-11413-4
Baldauf HM, Stegmann L, Schwarz SM, Ambiel I, Trotard M, Martin M, Burggraf M, Lenzi GM, Lejk H, Pan X, Fregoso OI, Lim ES, Abraham L, Nguyen LA, Rutsch F, König R, Kim B, Emerman M, Fackler OT, Keppler OT. Vpx overcomes a SAMHD1-independent block to HIV reverse transcription that is specific to resting CD4 T cells. Proceedings of the National Academy of Sciences USA 114(10):2729-2734 (2017). https://doi.org/10.1073/pnas.1613635114
Schneider C, Oellerich T, Baldauf HM, Schwarz SM, Thomas D, Flick R, Bohnenberger H, Kaderali L, Stegmann L, Cremer A, Martin M, Lohmeyer J, Michaelis M, Hornung V, Schliemann C, Berdel WE, Hartmann W, Wardelmann E, Comoglio F, Hansmann ML, Yakunin AF, Geisslinger G, Ströbel P, Ferreirós N, Serve H, Keppler OT*, Cinatl J Jr.*. SAMHD1 is a biomarker for cytarabine response and a therapeutic target in acute myeloid leukemia. Nature Medicine 23(2):250-255 (2017). https://doi.org/10.1038/nm.4255
Baldauf HM+, Pan X.+, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, König R, Fackler OT*, Keppler OT*. SAMHD1 restricts HIV-1 infection in resting CD4+ T cells. Nature Medicine 18:1682-1687 (2012). https://doi.org/10.1038/nm.2964
Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C, Harter C, Bekeredjian-Ding I, Sertel S, Lasitschka F, Keppler OT. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proceedings of the National Academy of Sciences USA 108(33):13688-93 (2011). https://doi.org/10.1073/pnas.1101684108
Goffinet C, Allespach I, Homann S, Tervo HM, Habermann A, Rupp D, Oberbremer L, Kern C, Tibroni N, Welsch S, Krijnse-Locker J, Banting G, Kräusslich HG, Fackler OT, Keppler OT. HIV-1 antagonism of CD317 is species-specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host & Microbe 5:285-297 (2009). https://doi.org/10.1016/j.chom.2009.01.009
Goffinet C, Allespach I, Keppler OT. HIV-susceptible transgenic rats allow rapid preclinical evaluation of antiviral compounds targeting virus entry or reverse transcription. Proceedings of the National Academy of Sciences USA 104:1015-1020 (2007). https://doi.org/10.1073/pnas.0607414104