Top 10 Publications
Steiner TM, Lettl C, Schindele F, Goebel W, Haas R, Fischer W* & Eisenreich W* (2021) Substrate usage determines carbon flux via the citrate cycle in Helicobacter pylori. Mol Microbiol 116: 841 (doi:10.1111/mmi.14775). (*, corr. Authors)
Lettl C, Haas R & Fischer W (2021) Kinetics of CagA type IV secretion by Helicobacter pylori and the requirement for substrate unfolding. Mol Microbiol 116: 794 (doi: 10.1111/mmi.14772).
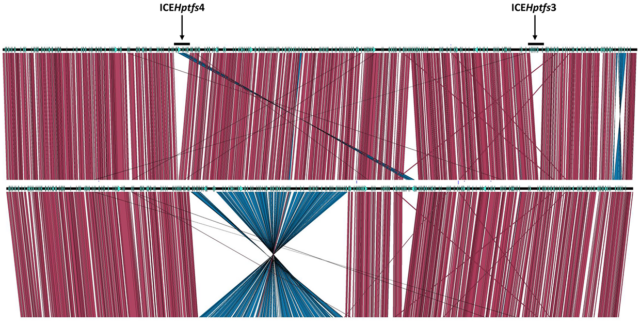
Weiss E, Spicher C, Haas R & Fischer W (2019) Excision and transfer of an integrating and conjugative element in a bacterial species with high recombination efficiency. Sci Rep 9: 8915 (doi: 10.1038/s41598-019-45429-z).
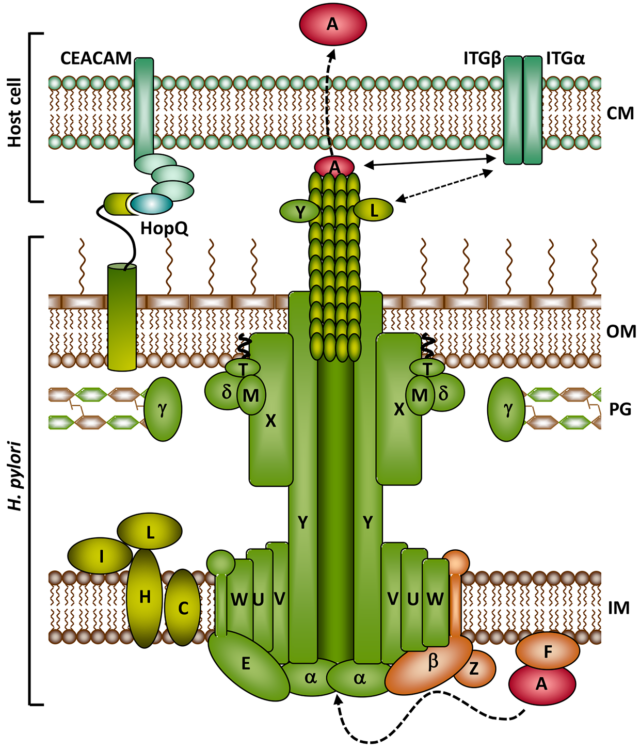
Zhao Q, Busch B, Jiménez-Soto LF, Ishikawa-Ankerhold H, Massberg S, Terradot L, Fischer W & Haas R (2018) Integrin but not CEACAM receptors are dispensable for Helicobacter pylori CagA translocation. PLoS Pathog 14: e1007359 (doi: 10.1371/journal.ppat.1007359).
Königer V, Holsten L, Harrison U, Busch B, Loell E, Zhao Q, Bonsor DA, Roth A, Kengmo-Tchoupa A, Smith SI, Mueller S, Sundberg EJ, Zimmermann W, Fischer W, Hauck CR & Haas R (2016) Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat Microbiol 2: 16188 (doi: 10.1038/nmicrobiol.2016.233).
Schindele F, Weiss E, Haas R & Fischer W (2016) Quantitative analysis of CagA type IV secretion by Helicobacter pylori reveals substrate recognition and translocation requirements. Mol Microbiol 100: 188 (doi: 10.1111/mmi.13309).
Jurik A, Haußer E, Kutter S, Pattis I, Praßl S, Weiss E & Fischer W (2010) The coupling protein Cagβ and its interaction partner CagZ are required for type IV secretion of the Helicobacter pylori CagA protein. Infect Immun 78: 5244.
Fischer W, Windhager L, Rohrer S, Zeiller M, Karnholz A, Hoffmann R, Zimmer R & Haas R (2010) Strain-specific genes of Helicobacter pylori: Genome evolution driven by a novel type IV secretion system and genomic island transfer. Nucleic Acids Res 38: 6089.
Hohlfeld S, Pattis I, Püls J, Plano GV, Haas R & Fischer W (2006) A C-terminal secretion signal is necessary, but not sufficient for type IV secretion of the Helicobacter pylori CagA protein. Mol Microbiol 59: 1624.
Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W & Haas R (2000) Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287: 1497.