Aktuelles
Diagnostics
Molecular Diagnostic
Background
Molecular biological detection methods have become established in many areas of medical microbiology. Specific DNA detection methods have made it possible to detect and identify bacteria without cultivation, to obtain information on resistance behaviour (e.g. vancomycin resistance in enterococci, methicillin resistance in S. aureus, clarithromycin resistance in H. pylori) or even to uncover infection chains in hospitals on a molecular level (e.g. spa typing of MRSA isolates, MLST for Legionella).
Molecular laboratory diagnostics of infectious diseases at the Max von Pettenkofer Institute
The molecular biological diagnostics of the Max von Pettenkofer Institute currently allow the direct PCR-based detection of approx. 35 human pathogens (including e.g. Borrelia, Mycoplasma). Using a so-called “universal” PCR, we are also able to detect DNA from more than 99% of human pathogens and identify them by subsequent sequencing.
This is particularly useful when pathogens are difficult or impossible to cultivate or when antibiotic therapy has already taken place before the sample was taken (e.g. apparently “sterile” meningitis, pleurisy or joint effusions). Another field of molecular diagnostics is the rapid detection of “atypical” pneumonia pathogens, such as Mycoplasma pneumoniae, Chlamydophila pneumoniae or Legionella. The rapid detection of MRSA and VRE is also part of molecular diagnostics.
Most PCR examinations are carried out daily, in many cases a result is obtained on the same day. Molecular diagnostics at the Max von Pettenkofer Institute is of course carried out according to the highest quality standards and has been subject to strict internal and external controls for years. The laboratory division “Molecular Diagnostics” is accredited by the Central Office of the Federal States for Health Protection with regard to Medicinal Products and Medical Devices (ZLG).
You can find further information in our list of services.
Diagnostics
Parasitology
Range of services
Microscopic examination of biopsies, punctates, secretions for protozoa and helminths.
Echinococcus granulosus serology.
For parasitological stool examination we recommend sending 3 stool samples from different days. The stool samples for parasitological examinations should be filled into special sample containers with stabiliser (MIF Fixativ) (stool: at least the size of a hazelnut; duodenal juice: at least 1 ml of sediment; 24-h collection urine). Sample containers with stabiliser can be ordered from the Max von Pettenkofer Institute (089/2180-72811).
Attention: MIF solution has a disinfecting effect. For this reason, the prepared sample material is no longer suitable for cultures (e.g. bacteriology).
You can find further information in our List of Services.
Diagnostics
Varia
The “Varia” section examines various patient samples, such as blood cultures, urine, materials from the respiratory tract, smears and tissue samples of all kinds from all body regions, as well as stool samples.
The examination programme includes all major aerobic and anaerobic bacteria as well as yeasts and moulds.
In the diagnostic area “Varia”, all conventional methods of bacteriological diagnostics are offered. These include microscopy, also as rapid diagnostics, cultural cultivation under aerobic and anaerobic conditions, identification of pathogens and sensitivity testing of relevant bacteria against all antibiotics listed in the KUM drug list. On special request, other antibiotics can of course also be included in the testing.
In addition, antigen detection is offered as rapid tests for legionella, pneumococci and meningococci as well as C. difficile toxin detection.
The Varia department also carries out the entire mycological diagnostics. Here, too, microscopy, culture, identification of yeasts and moulds and sensitivity testing of yeasts and, on special request, of moulds against the essential antimycotics are offered.
You can find the detailed information in our List of Services.
Of course, the medical staff of this unit are always available for consultation on diagnostic and therapeutic issues.
Diagnostics
Clinical microbiology - Antiinfectives consultation
In addition to comprehensive analysis, we offer advice on the rational and effective use of microbiological diagnostics (diagnostic stewardship) as well as assistance in interpreting findings and in anti-infective therapy. We support the Antibiotic Stewardship (ABS) initiative and work in an interdisciplinary team consisting of infectiologists, pharmacists and ABS certified microbiologists. Our goal is to achieve optimal clinical treatment outcomes for patients, avoid unnecessary toxicities and have a favourable impact on the development of resistance and costs through evidence-based therapy recommendations. Our guideline-based therapy recommendations take into account the local germ frequency and resistance statistics collected by the Max von Pettenkofer Institute at the University Hospital of Munich (KUM). Consultation takes place within the framework of regular or occasion-related microbiological / ABS rounds and by telephone on weekdays from 8:00 a.m. to 5:00 p.m. as well as outside office hours via the microbiological service telephone. Case discussions take place in cooperation with the clinical infectious diseases department in the infection colloquium on Mondays from 13:00 to 14:00. Written recommendations for the therapy of defined infectious disease patterns are drawn up by the infectious diseases colloquium at the KUM (iKUM) in a clinic-wide consensus and are continuously updated.
Links
ABS Initiative Deutschland
Antiinfektiva Surveillance
Die Bayerische Antibiotikaresistenz-Datenbank BARDa
interne Links
Stabstelle ABS (derzeit nur aus dem Intranet abrufbar)
Apotheke KUM
Stabstelle klinische Mikrobiologie und Hygiene
Sektion Infektiologie Med IV
Diagnostics
Clinical Microbiology- Infectious Serology
Background
Serological tests are of great importance in the differential diagnosis of many infectious diseases. The prerequisite is an intact immune system that is capable of producing antibodies when confronted with a pathogen. Since this usually takes a few days, serology is not suitable for acute diagnosis. In general, IgM detection correlates with a recent infection, while IgG antibodies are indicative of a chronic or past infection. A seroconversion or a significant rise in titer of more than 2 levels also speaks for an acute disease event. Therefore, a second examination of the serum is usually indicated.
Serological examinations can be disturbed by cross-reactions (antigen communities of different pathogens) or by polyclonal immune stimulation (e.g. in EBV infection). Autoantibodies such as rheumatoid factors can lead to false positive IgM detections. Therefore, serological findings should always be evaluated in conjunction with the clinical picture and other microbiological examinations and are usually not suitable as the sole basis for a diagnosis.
Serological examinations require approx. 5-10 ml of blood (serum tube) or 1 ml of cerebrospinal fluid (sterile tube). For the determination of the CSF/serum index, CSF and serum should be sent from the same day of collection.
If you have any questions, please contact us at the following telephone number:
Serology Main Laboratory: +49 89 2180-72840
Diagnostics
Tuberculosis diagnostics
Background
Mycobacteria are slow-growing bacteria whose cultural cultivation requires special conditions. Mycobacteria include the tuberculosis pathogens (M. tuberculosis, M. bovis, M. caprae, M. africanum, M. microti, M. canetii, M. pinnipedii), which are grouped together as the Mycobacterium tuberculosis complex, as well as more than 100 other mycobacterial species that are widely distributed in the environment. The tuberculosis laboratory at the Max von Pettenkofer Institute offers tests for the detection of the Mycobacterium tuberculosis complex as well as atypical mycobacteria. The pathogen is usually detected from sputum, bronchial secretion or tracheal secretion, but is also possible from gastric juice, urine, pleural exudate, cerebrospinal fluid and other puncture samples or biopsy samples.
For rapid clarification of infectivity, a microscopic examination for acid-fast rods (Ziehl-Neelsen stain) should always be carried out in cases of suspected pulmonary tuberculosis. About 10^4 germs/ml must be present to obtain a positive result, and a reliable differentiation between tuberculosis bacteria and environmental mycobacteria is not possible. Therefore, it is always necessary to do a culture as well. For the rapid diagnosis of Mycobacterium tuberculosis complex from patient samples, a molecular rapid detection by PCR is available (result is available within 1-2 working days). If the result is negative, however, infection with mycobacteria cannot be ruled out. After successful treatment of tuberculosis, DNA can sometimes still be detected after a year. At the Max von Pettenkofer Institute, a combination of liquid and solid media is used after decontamination of the test material. By using a liquid medium with indicators for bacterial growth, the detection time is reduced to 1 – 2 weeks. However, the cultures are incubated for up to 6 weeks (liquid media) to exclude delayed growth.
Non-tuberculous mycobacteria can also be detected culturally in our laboratory. As these are increasingly isolated, especially in immunocompromised or cystic fibrosis patients, reliable and rapid detection is also an important part of our diagnostics. Resistance tests for non-tuberculous mycobacteria are not part of our range of services. In this case, please contact the National Reference Centre for Mycobacteria in Borstel (Prof. Dr. med. Florian Maurer; Nat. Reference Centre Tel: 04537 – 188 – 2110). Reference is made to the ATS – Guidelines for the Treatment of Non-Tuberculous Mycobacterial Infections of the American Thoracic Society and the recommendations of the DZK – Central Committee for the Control of Tuberculosis.
If you have any other questions, you can reach us at the following telephone number: Tuberculosis Laboratory: +49 89 2180-72844
Range of services
- Microscopic detection of acid-fast rods
- Culture cultivation of mycobacteria with liquid and solid culture media
- Detection of Mycobacterium tuberculosis complex with nucleic acid amplification methods
- Differentiation of mycobacteria with conventional methods and molecular biological techniques
- Susceptibility testing of Mycobacterium tuberculosis complex in liquid culture media
Useful information on diagnostics (briefly summarised)
A test of 3 sputums taken on consecutive days can exclude overt tuberculosis with a very high probability in the case of culturally negative findings.
If pulmonary tuberculosis is suspected: BAL, sputum (if overt TB) or gastric juice.
If extrapulmonary tuberculosis is suspected: Biopsy, punctate or cerebrospinal fluid.
Open tuberculosis is said to exist if there is at least one cultural detection of MTB complex from a material that is excreted by the body (e.g. urine or sputum).
Emergency preparation
A so-called native preparation without pre-enrichment for rapid diagnosis is not recommended, because the diagnostic sensitivity decreases significantly due to loss of patient material. If no acid-fast rods are detected in it, this by no means excludes a tuberculosis infection.
You can find more information in our List of Services.
Diagnostics
SARS-CoV-2 diagnostics
For the highly sensitive, molecular detection of SARS-CoV-2, we perform PCR-based, quantitative detection of the pathogen from respiratory swab material seven days a week. Both rapid diagnostics (result approx. 1-2 hours after sample receipt) and stand tests (result approx. 4-6 hours after sample receipt) are available.
Furthermore, we routinely perform quantitative determinations of antibodies against the spike antigen (vaccination success) and the nucleocapsid antigen (serological detection of infection) of SARS-CoV-2.
In research projects, we continue to perform neutralization activity assay against replication-competent variants of SARS-CoV-2 (EU1, as well as variants-of-concern (VoCs) alpha, beta, gamma, delta, and omicron-BA.1, BA.4, BA.5) and other current VoCs or variants-of-interest (VoIs). We also quantify the avidity of anti-spike IgGs to better assess not only the quantity but also the quality of these important antibodies for protection against infection and disease.
Diagnostics
Hygiene
The hygiene laboratory is approved as a drinking water testing centre according to §15 TrinkwV and carries out hygienic-microbiological examinations of water samples for both hospitals and private senders. In addition, we offer a wide range of hygienic-microbiological examinations for quality assurance in medical facilities (especially in clinics and medical or dental practices).

Virology
Several research groups are working on a broad range of projects ranging from basic science to translational studies related to important viral human pathogens, in part with the support of the Institute’s diagnostics team. The chair of virology is Professor Oliver T. Keppler, MD.
The current focus areas comprise:
- Viral persistence and immune destruction by HIV
- Viral evolution and immune evasion of SARS-CoV-2
- Antiviral strategies against HIV and SARS-CoV-2
- Species-specific aspects of innate immunity and HIV small animal model development
- Interaction of CMV with the immune system
- High-throughput screening technologies to identify virus-host interactions in human coronaviruses
- SAMHD1-dependent chemoresistance and viral replication
- National Reference Center for Retroviruses
- Bay-VOC: Molecular genetic SARS-CoV-2 surveillance network in Bavaria
- FOR-COVID: Bavarian research network on COVID-19
Our research projects are funded by the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF), the German Center for Infection Research (DZIF), the Bavarian State Ministry of Health and Care, the Bavarian State Ministry of Science and Art, the Bavarian Research Foundation, the Wilhelm Sander Foundation, and other foundations.
Virology
Research Group PD Dr. rer. nat. Dr. habil. med. Albrecht von Brunn
The von Brunn laboratory investigates and identifies protein-protein interactions of zoonotic viruses at intra-viral and virus-host levels. Goal is to identify fundamental processes and pathways for interference with viral replication in infected host cells leading to the development of broad-spectrum antivirals. Main focus is put on coronaviruses (including mildly pathogenic “common cold” viruses and highly pathogenic SARS-CoV/SARS-CoV-2 and MERS-CoV) and Zika virus. The strategy contributes to pre-pandemic and pandemic preparedness against emerging viral infections.
Diagnostics
List of pathogens
Teaching

Teaching at the Max von Pettenkofer Institute
There is no doubt that infectious diseases caused by viruses, bacteria, fungi and parasites are of great social importance. In this regard, the interdisciplinary education of nearly 1000 students of the next generation of human physicians, dentists, pharmacologists and biologists annually at our institute plays an important role.
We strive to impart knowledge at the interface of disease patterns, state-of-the-art diagnostics, and cutting-edge international research through a blend of proven and innovative teaching formats.
Students of human medicine are taught microbiology, virology, hospital hygiene and immunology through traditional lectures, practicals, seminars as well as interactive training such as inverted classroom, hybrid lectures and online courses with case presentations in the 1st clinical year (5th semester).
Students of dental medicine in the 2nd study section participate in traditional lectures with a focus on prevention of nosocomial infections.
The education of pharmacy students consists of a lecture part and a laboratory practical. The focus here is on the effects of antiviral, antibiotic and immunomodulatory therapies.
Students in the elite Master’s Program in Human Biology complete a lecture cycle and laboratory practicals with a focus on diagnostics, pathogenesis, and therapeutic targets in infectious diseases.
Students in the Biochemistry and Molecular & Cellular Biology Master’s Programs and the Elite Master’s Program Human Biology, as well as undergraduate students in Biology and Computer Science, participate in the “Molecular Virology” lecture series and the “Advances in Molecular Virology” seminar. For a complete module of the Master’s programs, additional 4- to 8-week laboratory practicals are offered.
Public relations is another important component of knowledge transfer with broad impact in infection research. We implement this through interviews, public lectures and focal events, e.g. on World AIDS Day, for schoolchildren.
There is also the possibility to complete a clinical traineeship or a tertial in hygiene/microbiology and virology as part of the practical year.
Teaching
Teaching in Medical Microbiology
Welcome to the courses offered by the Chair of Medical Microbiology and Hospital Hygiene at the Max von Pettenkofer Institute.
You can find an overview of the individual courses by clicking on the tabs below for the respective courses (human medicine, practical year, dentistry, pharmacy and biology).
For LMU students, current dates and venues as well as further information can be accessed via MedMoodle.
Teaching
Lehre in der Virologie
Herzlich willkommen bei den Lehrangeboten des Lehrstuhls für Virologie am Max von Pettenkofer-Institut.
Einen Überblick über die einzelnen Lehrveranstaltungen finden Sie über die untenstehenden Reiter zu den jeweiligen Lehrangeboten (Humanmedizin, Praktisches Jahr, Zahnmedizin und Pharmazie).
Für Studierende der LMU sind aktuelle Termine und Veranstaltungsorte sowie weitere Informationen unter MedMoodle zugänglich.
Medical Microbiology and Hospital Epidemiology
Research Group Prof. Dr. Christine Josenhans
The group led by Prof. C. Josenhans joined the Max von Pettenkofer Institute in the fall 2017.
The gastrointestinal tract of humans and most vertebrate organisms is at the same time the largest body surface, colonization niche of a variety of different microbes, as well as the most important barrier and entry point of gastrointestinal and some systemic pathogens. Gastrointestinal pathogens, in particular bacteria, are adapted in a specific manner to the mucosal niche of the gut and stomach lining. They assemble complex secretion systems, which help them to be motile within the mucus, and establish contact and interactions with the host and local host cells. Likewise, they possess metabolic and other factors, which enable them to compete with the resident microbiota of the gut and surmount colonization resistance. In addition, they are able, with the help of specific factors and functions, to influence the composition of the resident microbiota and the inflammatory response of the local tissues, in order to govern their own temporal and spatial persistence within the niche.
Major scientific interest in the Josenhans lab is on the host innate immune response towards gastrointestinal pathogens and its modulation. In this context, the modulatory activity of bacterial complex secretion systems (type 3, type 4, type 6) on the host cell is investigated, including the biochemical and structural characterization of its protein components. The lab also addresses questions concerned with gene and protein regulation and modification in host and pathogen, also during the infectious process. A second focus is placed on host modulation by pathogens and the modulation of inflammatory diseases, also with respect to environmental factors, including the resident gastrointestinal microbiota. Main model systems to study chronic and acute gastrointestinal bacterial pathogens are the human pathogens Helicobacter pylori and Campylobacter jejuni as well as the chronic mouse gut pathogen Helicobacter hepaticus, which can serve as a model for chronic inflammatory diseases. We also place a major emphasis on the factors that activate and modulate the host innate immune system (human but also others), and, on the host side, the receptors and signal transduction pathways that are invoved in the host innate immune response.
Medical Microbiology and Hospital Epidemiology
Research Group Prof. Dr. med. Sebastian Suerbaum
Our group performs both basic and translational research aiming at better understanding, diagnosing, treating and preventing infections of the gastrointestinal tract. Our main focus is on the human gastric carcinogen, Helicobacter pylori, where we use a combination of experimental approaches and comparative genomics to understand the genetic variability of H. pylori and its role in adapting the pathogen to its human host. Other projects study the genome of the most important human diarrheal pathogen in Germany, Campylobacter jejuni, the mouse pathobiont, Helicobacter hepaticus and its role in inflammatory bowel disease, and the role of the intestinal and gastric microbiota in health and disease.
Medical Microbiology and Hospital Epidemiology
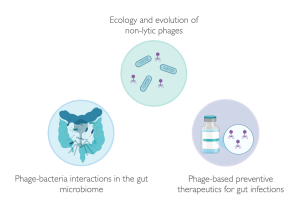
Research group Prof. Dr. Carolin Wendling
Our group aims to unravel how bacteria evolve in response to abiotic and biotic environmental interactions. We thereby focus on one of the most important players that influence bacterial ecology and evolution: mobile genetic elements (MGEs), including plasmids and phages, which can provide their bacterial hosts with accessory genes, such as virulence and/ or antibiotic resistance genes. By working at different levels of biological complexity, from clonal populations to complex microbial communities, we aim to elucidate phage-bacteria interactions in diverse backgrounds. Utilizing a multidisciplinary approach, we employ a diverse array of techniques such as molecular biology, experimental evolution, in vivo infection experiments, genomics, and mathematical modelling. This holistic approach enables us to explore antimicrobial resistance, infectious diseases, and phage therapy from various angles, leading to a comprehensive understanding of these complex phenomena and advancing phage therapy applications in gut infections.
Our research priorities include the following:
- Understanding ecological and evolutionary interactions between prophages, commensals, and pathogens in the gastro-intestinal tract
- Reveal the role of prophages in gut health and diseases
- Explore how novel insights into phage ecology and evolution can improve phage therapy
Medical Microbiology and Hospital Epidemiology
Structured doctorate program
The Max von Pettenkofer Institute (MvPI) of LMU offers a new structured international PhD program (starting winter semester 2019/2020) called “Infection Research on Human Pathogens@MvPI” under the umbrella of MMRS (https://www.graduatecenter.uni-muenchen.de/promotionsprogramme/index.html). This PhD program combines the work on an individual PhD project (natural science or medical PhD thesis) in the field of infection research (with focus on microbiology/bacterial pathogens, microbiota research, virology and infectious immunology) with program-specific side events for in-depth and comprehensive thematic rounding in central and current topics of infection research as well as further practical courses and offers on transferable skills.
Information on new projects and currently offered positions in the program can be found on the MvPI website (https://www.mvp.uni-muenchen.de/stellenangebote). Further information about the institute, the main topics worked on there and the current working groups can also be found on our institute website or can be obtained from the secretariat responsible for the PhD program at MvPI, Ms. Constanze Hartmannsgruber, Tel: 089-2180 72805 Email: PhD.infection(at)mvp.lmu.de).
We are currently in the process of adding a time-bound formal application procedure. Until this is done, please contact the professors and group leaders directly if you are interested in a PhD for your unsolicited application and check our “Job Opportunities” page regularly. You can also send your complete unsolicited application with two references to our office at any time (email). Thank you for your interest.
Virology
Research Group PD Dr. rer. nat. Barbara Adler
Human cytomegalovirus (HCMV) infections are a major cause of morbidity and mortality in immunocompromised humans like transplant patients or the unborn or prematurely born child. Currently available anti-HCMV treatments are very costly, associated with severe side effects, and the development of drug resistance. A licensed vaccine to protect from HCMV infection does not exist. Common targets for antiviral therapy are for example envelope glycoproteins, which promote virus entry. Our research focus is CMV gHgL glycoprotein complexes. We study mechanisms of gHgL glycoprotein-dependent entry, the role of these complexes in virus infections, their potential as vaccine antigens, and when using CMV as a vaccine vector, their role in shaping the vaccine-induced immune response.
Virology
Research Group PD Dr. rer. nat. Hanna-Mari Baldauf
The VIIRAL lab focuses on species-specific innate immunity in the context of retroviral infections and therapeutic options for acute leukemia. For the first focus, we use different model systems ranging from simple retroviruses to complex lentiviruses, like HIV, in order to obtain more insight into species-specific differences in innate immunity. Building on our previous work on antiviral host cell factors as well as our observation that rabbits as a species display fewer blocks to HIV replication than mice or rats, we seek to develop a fully permissive transgenic rabbit model to facilitate the development of novel strategies for the prevention or treatment of HIV/AIDS. For the second focus, we are studying novel therapeutic options for acute leukemia based on our previous work with antiviral proteins relevant also in the cancer field.
Virology
Research Group Prof. Dr. med. Oliver T. Keppler
The Keppler laboratory investigates fundamental principles of virus-host interactions, in particular for the pandemic pathogens HIV and SARS-CoV-2, as well as specific mechanisms of resistance to chemotherapy in different malignancies. Using state-of-the-art technology and primary model systems, we seek to unravel the immunodestruction induced by HIV and the ability of the virus to infect and establish latency in resting CD4 T cells. In the fight against SARS-CoV-2, we are testing novel antiviral approaches and aiming to identify drugable cellular pathways by genetic and high-throughput screening approaches. At the interface of virology and oncology we are studying the SAMHD1-dependent resistance to nucleoside analog-based chemotherapy and developing SAMHD1-targeted strategies to expand therapeutic options for hard-to-cure hematological and solid tumors.
Virology
Research Group PD Dr. med. Maximilian Muenchhoff
Our research work is devoted to understanding host-virus interactions of the two pandemic pathogens HIV-1 and SARS-CoV-2. Building on our platform for genomic surveillance of SARS-CoV-2 we are investigating its evolution in adaptation to the human immune system. We seek to elucidate immune escape mutations that are selected under antibody and T-cell responses in emerging variants and in hosts with prolonged infection. With a broad interest in the immunopathogenesis of viral infections we aim to decipher immunological signatures that are associated with differential disease phenotypes in clinical cohorts of SARS-CoV-2- and HIV-1-infected individuals. At the National Reference Center for Retroviruses we are developing state-of-the-art methods to quantify and characterize HIV persistence in patient samples and primary cell models.
Contact
Chair of Medical Microbiology and Hospital Epidemiology
Constanze Hartmannsgruber, M.A.
Assistant Prof. Sebastian Suerbaum
80336 München
Virology
Brigitte Held, Dipl.-Angl. BWL
Assistant to Prof. Oliver T. Keppler
80336 München
Gate
Telephone Exchange
80336 München